The Green Smoothie Health Fad: This Road to Health Hell is Paved with Toxic Oxalate Crystals.
By William Shaw Ph.D.
Oxalates as a major cause of cardiovascular disease and many other chronic diseases and as a target for intravenous EDTA therapy.
Recent internet news indicated the conviction for attempted murder of an oncologist who attempted to kill her boyfriend who was also involved with another woman. The attempted murder weapon was ethylene glycol, popularly known as antifreeze, which had been placed in his coffee just after coitus. Although emergency measures saved the boyfriend’s life, extensive deposits of oxalate crystals, the main toxic metabolite of ethylene glycol, had caused extensive kidney and liver damage, reducing the man’s lifespan by about half.
Achieving similar results in sabotaging your own health can now be achieved by a popular concoction called a “green smoothie”. A recent Google search for “green smoothie” yielded 609,000 hits. In addition, a recent “improving your diet” seminar I attended promoted this same idea. Interestingly, on the same day, I reviewed test results on a urine organic acid test of a woman with oxalate values three times the upper limit of normal. A conversation with the patient indicated that she had recently turned to consuming daily “green smoothies” to “clean up her diet”. The most common “green” component of the most popular green smoothies are spinach, kale, Swiss chard, and arugula. Each of these greens is loaded with oxalates. A typical internet recipe advises that two cups of packed raw spinach leaves is a good starting point for a good smoothie. In addition to the high oxalate greens added to the blender to produce the green smoothie, smoothie proponents frequently recommend the additions of a variety of berries or almonds, also containing high oxalate amounts. Similar high urine oxalate results were found in organic acid tests of a number of patients with kidney stones who had decided to eat large spinach salads daily as a “move to clean up my unhealthy diet”. Unfortunately kidney stones are not the only health problems the people with the green smoothies and large spinach salads will experience with their new “clean” diet.
Seventy-five years ago, a food scientist of the Campbell Soup Company (1) reported:
“Only a few foods, notably spinach, Swiss Chard, New Zealand spinach, beet tops, lamb’s quarter, poke, purslane, and rhubarb have high oxalate content. In them, expressed as anhydrous oxalic acid, it is often considerably over 10% on a dry basis. In fifty-three samples, including practically all commercial and many experimental varieties grown in California and in Maryland as well as those shipped from Texas, Florida and Carolina, the average anhydrous oxalic acid content was 9.02% on the dry basis (maximum 12.6, minimum 4.5).
- It is apparent from data in tables 2, 3 and 4 that whereas spinach greatly increases the calcium content of the low calcium but well performing basal diet, it decidedly interferes with both growth and bone formation. If to a diet of meat, peas, carrots and sweet potatoes, relatively low in calcium but permitting good though not maximum growth and bone formation, spinach is added to the extent of about 8% to supply 60% of the calcium, a high percentage of deaths occurs among rats fed between the age of 21 and 90 days. Reproduction is impossible. The bones are extremely low in calcium, tooth structure is disorganized and dentine poorly calcified. Spinach not only supplies no available calcium but renders unavailable considerable of that of the other foods. Considerable of the oxalate appears in the urine, much more in the feces.”
The author also discovered that, in addition, to leading to excessive death, defective reproduction, high oxalate foods also cause soft and pliable bones and defective teeth.
Oxalate and its acid form oxalic acid are organic acids that are primarily from three sources: the diet, from fungus infections such as Aspergillus and Penicillium and possibly Candida (2-10), and also from human metabolism (11).
Oxalic acid is the most acidic organic acid in body fluids and is used commercially to remove rust from car radiators. Antifreeze (ethylene glycol) is toxic primarily because it is converted to oxalate. Two different types of genetic diseases are known in which oxalates are high in the urine. The genetic types of hyperoxalurias (type I and type II) can be determined from the organic acid test done at The Great Plains Laboratory. Foods especially high in oxalates include spinach and similar leafy vegetables, beets, chocolate, peanuts, wheat bran, tea, cashews, pecans, almonds, berries, and many others. Oxalates are not found in meat or fish at significant concentrations. Daily adult oxalate intake is usually 80-120 mg/d; it can range from 44-1000 mg/d in individuals who eat a typical Western diet. I estimate that the person who consumes a green smoothie with two cups (about 150 grams) of spinach leaves is consuming about 15 grams or 15,000 mg oxalates or about 150 times the average daily oxalate intake. A complete list of high oxalate foods is available on the Internet at http://www.upmc.com/patients-visitors/education/nutrition/pages/low-oxalate-diet.aspx
High oxalate in the urine and plasma was first found in people who were susceptible to kidney stones. Most kidney stones are composed of calcium oxalate. Stones can range in size from the diameter of a grain of rice to the width of a golf ball. It is estimated that 10% of males may have kidney stones some time in their life. Because many kidney stones contain calcium, some people with kidney stones think they should avoid calcium supplements.
However, the opposite is true. When calcium and magnesium are taken with foods that are high in oxalates, oxalic acid in the intestine combines with calcium and magnesium to form insoluble calcium and magnesium oxalate crystals that are eliminated in the stool. These forms of oxalate cannot be absorbed into the body. When calcium and/or magnesium are low in the diet, oxalic acid is soluble in the liquid portion of the contents of the intestine (called chyme) and is readily absorbed from the intestine into the bloodstream. If oxalic acid is very high in the blood being filtered by the kidney, it may combine with calcium and other metals, including heavy metals like lead and mercury, to form crystals that may block urine flow, damage the kidney and cause severe pain.
However, such crystals may also form in the bones, skin, joints, eyes, thyroid gland, blood vessels, lungs, and even the brain (11-14). In addition, oxalate crystals in the bone may crowd out the bone marrow cells, leading to anemia and immunosuppression (14). In addition to autism and kidney disease, individuals with fibromyalgia and women with vulvar pain (vulvodynia) may suffer from the effects of excess oxalates (15-18).
Recent evidence also points to the involvement of oxalates in stroke, atherosclerosis, and in endothelial cell dysfunction (19-21). High amounts of oxalates were found concentrated in atherosclerotic lesions of the aortas and coronary arteries of a number of individuals at autopsy; these individuals did not have oxalate deposits in the kidney but did have oxalate deposits in other organs such as the thyroid gland and testis. Since the stains used by most pathologists examining atherosclerotic lesions cannot readily determine the presence of oxalates in diseased arteries, it seems possible that this cause of atherosclerosis may be much more common than previously realized. I suspect that this oxalate-induced atherosclerosis is a much more common cause of atherosclerosis than high cholesterol. Furthermore, since EDTA is effective in the removal of oxalate crystals deposited in the tissues (22,23), the benefits of intravenous EDTA in the treatment of cardiovascular disease may be mediated largely by the removal of oxalate crystals and their associated heavy metals from the tissues in which they are deposited.
Oxalate crystals may cause damage to various tissues. The sharp crystals may cause damage due to their physical structure and may also increase inflammation. Iron oxalate crystals may also cause significant oxidative damage and diminish iron stores needed for red blood cell formation (11). Oxalates may also function as chelating agents and may chelate many toxic metals such as mercury and lead. However, unlike common chelating agents like EDTA and DMSA that cause metals to be excreted, a reaction of oxalate with heavy metals like mercury and lead leads to the precipitation of the heavy metal oxalate complex in the tissues, increasing the toxicity of heavy metals by delaying their excretion (24).
What steps can be done to control excessive oxalates?
- Use antifungal drugs to reduce yeast and fungi that may be causing high oxalates. Children with autism frequently require years of antifungal treatment. I have noticed that arabinose, a marker used for years for yeast/fungal overgrowth on the organic acid test at The Great Plains Laboratory, is correlated with high amounts of oxalates (Figure 1). Candida albicans produces high amounts of the enzyme collagenase (25), which breaks down collagen in the gastrointestinal tract to form the amino acid hydroxyproline, which in a series of reactions is converted to oxalates, especially in people with low vitamin B6 Candida organisms have been found surrounding oxalate stones in the kidney (10).
- Give supplements of calcium citrate and magnesium citrate to reduce oxalate absorption from the intestine. Citrate is the preferred calcium form to reduce oxalate because citrate also inhibits oxalate absorption from the intestinal tract. The best way to administer calcium citrate would be to give it with each meal. Children over the age of 2 need about 1000 mg of calcium per day. Of course, calcium supplementation may need to be increased if the child is on a milk-free diet. The most serious error in adopting the gluten-free, casein-free diet is the failure to adequately supplement with calcium.
- Give chondroitin sulfate to prevent the formation of calcium oxalate crystals (26).
- Vitamin B6 is a cofactor for one of the enzymes that degrade oxalate in the body and has been shown to reduce oxalate production (27).
- Increase water intake to help to eliminate oxalates.
Measuring oxalate toxicity
The organic acid test (Table 1) is one of the best measures for determination of both genetic and nutritional factors that lead to toxic oxalates. The organic acid test includes two additional markers, glycolic and glyceric acids, that are markedly elevated in genetic causes of excessive oxalate, the hyperoxalurias I and II. In addition, the organic acid includes factors such as high fungal and Candida markers that make oxalate (fungus) or their precursors (Candida). Finally, although vitamin C poses little risk of excess oxalates at doses up to 2000 mg per day, I have measured with the organic acid test marked increases in oxalates (more than ten times the upper limit of normal) in a child with impaired kidney function after a 50,000 mg intravenous vitamin C megadose. The organic acid test also includes the main vitamin B6 metabolite pyridoxic acid that diminishes the body’s own production of oxalates.
Published in the January 2015 issue of The Townsend Letter
References
- KOHMANI,EF. OXALIC ACID IN FOODS AND ITS BEHAVIOR AND FATE IN THE DIET Journal of Nutrition 18(3):233-246,1939
- Tsao, G. Appl Microbiol. 1963 May; 11(3): 249-254. Production of Oxalic Acid by a Wood-Rotting Fungus.
- Takeuchi H, Konishi T, Tomoyoshi T. Observation on fungi within urinary stones. Hinyokika Kiyo. 1987 May;33(5):658-61.
- Lee SH, Barnes WG, Schaetzel WP. Pulmonary aspergillosis and the importance of oxalate crystal recognition in cytology specimens. Arch Pathol Lab Med. 1986 Dec;110(12):1176-9.
- Muntz FH. Oxalate-producing pulmonary aspergillosis in an alpaca. Vet Pathol. 1999 Nov;36(6):631-2.
- Loewus FA, Saito K, Suto RK, Maring E. Conversion of D-arabinose to D-erythroascorbic acid and oxalic acid in Sclerotinia sclerotiorum. Biochem Biophys Res Commun. 1995 Jul 6;212(1):196-203.
- Fomina M, Hillier S, Charnock JM, Melville K, Alexander IJ, Gadd GM. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl Environ Microbiol. 2005 Jan;71(1):371-81.
- Ruijter, G.J.G., van de Vondervoort, P.J.I. & Visser, J. (1999) Oxalic acid production by Aspergillus niger: an oxalate-non-producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology 145, 2569–2576.
- Ghio AJ, Peterseim DS, Roggli VL, Piantadosi CA. Pulmonary oxalate deposition associated with Aspergillus niger infection. An oxidant hypothesis of toxicity. Am Rev Respir Dis. 1992 Jun;145(6):1499-502.
- Takeuchi H, Konishi T, Tomoyoshi T. Detection by light microscopy of Candida in thin sections of bladder stone. Urology. 1989 Dec;34(6):385-7.
- Ghio AJ, Roggli VL, Kennedy TP, Piantadosi CA. Calcium oxalate and iron accumulation in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000 Jun;17(2):140-50.
- Ott SM, Andress DL, Sherrard DJ. Bone oxalate in a long-term hemodialysis patient who ingested high doses of vitamin C. Am J Kidney Dis. 1986 Dec;8(6):450-4.
- Hall BM, Walsh JC, Horvath JS, Lytton DG. Peripheral neuropathy complicating primary hyperoxaluria. J Neurol Sci. 1976 Oct;29(2-4):343-9.
- Sahin G, Acikalin MF, Yalcin AU. Erythropoietin resistance as a result of oxalosis in bone marrow. Clin Nephrol. 2005 May;63(5):402-4.
- Sarma AV, Foxman B, Bayirli B, Haefner H, Sobel JD. Epidemiology of vulvar vestibulitis syndrome: an exploratory case-control study. Sex Transm Infect. 1999 Oct;75(5):320-6.
- http://wisewitch.blogspot.com/2006/07/guaifenesinfibromyalgia-and-oxalates.html
- http://www.vulvarpainfoundation.org/Lowoxalatetreatment.htm
- Gregory A. Fishbein, , Robert G. Micheletti, Judith S. Currier, Elyse Singer, Michael C. Fishbein, Atherosclerotic Oxalosis in Coronary Arteries Cardiovasc Pathol. 2008 ; 17(2): 117–123. .
- RI Levin, PW Kantoff and EA Jaffe Uremic levels of oxalic acid suppress replication and migration of human endothelial cells. Arterioscler Thromb Vasc Biol 1990, 10:198-207
- Giuseppe Di Pasquale, , Mariangela Ribani, Alvaro Andreoli, , Gian Angelo Zampa, and Giuseppe Pinelli, Cardioembolic Stroke in Primary Oxalosis With Cardiac Involvement. Stroke 1989, 20:1403-1406.
- Ziolkowski F, Perrin DD. Dissolution of urinary stones by calcium-chelating agents: A study using a model system. Invest Urol. 1977 Nov;15(3):208-11.
- Burns JR, Cargill JG 3rd. Kinetics of dissolution of calcium oxalate calculi with calcium-chelating irrigating solutions. J Urol. 1987 Mar;137(3):530-3.
- http://www.greatplainslaboratory.com/home/eng/oxalates.asp (accessed Oct 16,2014)
- H Kaminishi, Y Hagihara, S Hayashi, and T Cho . Isolation and characteristics of collagenolytic enzyme produced by Candida albicans. Infect Immun. 1986 August; 53(2): 312–316.
- Shirane Y, Kurokawa Y, Miyashita S, Komatsu H, Kagawa S. Study of inhibition mechanisms of glycosaminoglycans on calcium oxalate monohydrate crystals by atomic force microscopy. Urol Res. 1999 Dec; 27(6):426-31.
- Chetyrkin SV, Kim D, Belmont JM, Scheinman JI, Hudson BG, Voziyan PA. Pyridoxamine lowers kidney crystals in experimental hyperoxaluria: a potential therapy for primary hyperoxaluria.
Figure 1. Summary of oxalate souces. Candida albicans has the ability to produce an enzyme called collagenase, an enzyme that breaks down collagen, a major human body component that makes up 30% of our proteins. In addition, ethylene glycol (antifreeze) is converted to oxalate by human enzymes. Many fungi, including those that infect humans, also produce oxalates directly and oxalate stones may be found in tissues like the lung and sinuses that are fungi-infected. Vitamin C (ascorbic acid), especially at doses of more than 2000 mg per day, can break down to form oxalates and certain fungi can convert the Candida byproduct arabinose to oxalate. Collagen produced by Candida albicans breaks down the major human protein collagen to form hydroxyproline,which is one of the major amino acids in collagen. Hydroxyproline is also a major constituent of gelatin, a popular dessert. Hydroxyproline can then be converted to glycolate and glyoxylate by a series of reactions in the human body. Further metabolism of glyoxylate is at a critical junction. If adequate amounts of vitamin B6 are available, the enzyme AGT converts glyoxylate to the amino acid glycine. If vitamin B6 is deficient, glyoxylate is increasingly converted to oxalate by lactate dehydrogenase although some of it is converted back to glycolate, which is again susceptible to forming additional oxalate.
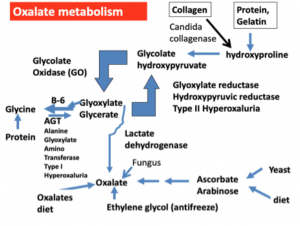
Table 1. Factors that affect oxalate metabolism that are covered in the urine organic acid test.
| Metabolites | Significance |
| Oxalic acid | Extremely acidic organic acid that traps heavy metals and deposits in a variety of tissues throughout the body |
| Pyridoxic acid | Major metabolite of vitamin B6- high amounts of vitamin B6 shunts oxalate precursors to the formation of glycine instead of oxalic acid |
| Glycolic acid | Byproduct of Candida produced when Candida enzyme collagenase converts hydroxyproline from collage to glycolic acid. It is found elevated in the genetic hyperoxaluria type I |
| Glyceric acid | . It is found elevated in the genetic hyperoxaluria type II |
| Arabinose, tartaric acid | Candida markers |
| 5-Hydroxy-methyl-furoic Acid, Furan-2,5-Dicarboxylic Acid | Metabolites of fungi such as Aspergillus that may produce oxalates directly |
| Ascorbic acid | High oral or intravenous intake may lead to excessive oxalate production |



